MBL Burden
Shining a light on MBL-producing bacteria: how they impact your patients and your hospitals
As the number of infections caused by carbapenemase-producing bacteria continues to grow, the burden on patients and healthcare systems is being seen throughout the world.1–4
This is especially true for infections caused by metallo-β-lactamase (MBL)-producing Enterobacterales. The majority of bacteria producing an MBL often co-produce other carbapenemases.5 This decreases the number of effective treatment options,6,7 thus significantly impacting patient outcomes and healthcare-resource utilisation, including:3,6,8–12
- Higher mortality rates
- Increased length of hospital and ICU stays
- Decreased likelihood to receive appropriate empiric treatment
- Longer durations of antibiotic therapy
- Delayed time to appropriate therapy
- Increased infection control measures
- Higher direct and indirect costs
The impact of carbapenemases, including MBLs, on patient outcomes:
Likely to receive active empiric therapy (p=0.07)†‡

Likely to receive active directed antibiotic therapy (p<0.001)§ǁ

Tamma PD , et al. 2017 : Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia.
14-day mortality:
CP-CRE

non-CP-CRE
Adjusted Odds Ratio for 14- and 30-day mortality
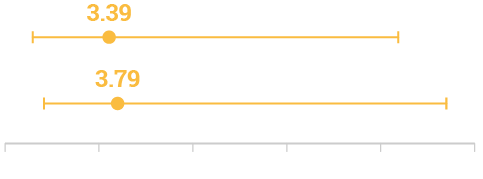
14-day mortality
Adjusted Odds Ratio (95% CI, 0.85–12.80; p=0.08)¶#
30-day mortality
Adjusted Odds Ratio (95% CI, 1.02–14.02; p=0.04)¶#
Mean total length of hospital stay (p<0.001)

Mean total length of ICU stay (p<0.001)

de Jager P , et al. 2015 : Nosocomial outbreak of New Delhi metallo-β-lactamase-1-producing Gram-negative bacteria in South Africa: A case-control study.
In-hospital mortality:

NDM-producing Enterobacterales

matched case controls
Adjusted Odds Ratio for in-hospital mortality††
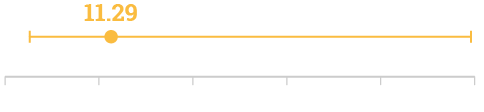
In-hospital mortality
Adjusted Odds Ratio (95% CI 2.57–49.60; p=0.001)††
Received appropriate empiric therapy§§

Daikos GL , et al. 2009 : Prospective observational study of the impact of VIM-1 metallo-β-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections.
In-hospital mortality:

VIM-positive K. pneumoniae

non-VIM-positive K. pneumoniae
Odds Ratio for all-cause 14-day mortality‡‡
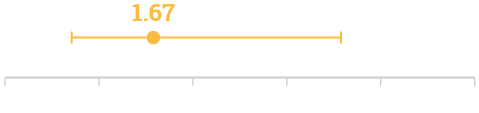
14-day mortality
Univariate analysis of factors (VIM-1 production): Odds Ratio for all-cause 14-day mortality (95% CI 0.76–3.68; p=0.20)‡‡
Received effective empiric therapy (p=0.061)
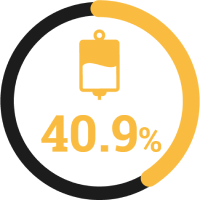
Hayakawa K , et al. 2020 : Comparison between IMP carbapenemase-producing Enterobacteriaceae and non-carbapenemase-producing Enterobacteriaceae: a multicentre prospective study of the clinical and molecular epidemiology of carbapenem-resistant Enterobacteriaceae.
30-day mortality:

IMP-producing Enterobacterales

matched case controls
Propensity score-adjusted analyses of clinical outcomes##
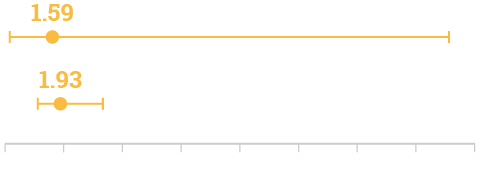
30-day mortality
(95% CI, 0.17–15.09; p=0.688)
Length of stay after isolation of IMP/non-IMP, days
(95% CI, 1.13–3.31; p=0.018)***
MBL-producing bacteria represent a serious concern to public health but what can be done to improve patient and healthcare-associated outcomes?
Patient profiles
Physicians have the power to improve patient outcomes
Learn about key patient risk factors associated with infections caused by MBL-producing Enterobacterales.

Epidemiology
Understanding local epidemiology is crucial in the fight against MBL-producing pathogens13,14
Explore MBL epidemiology and learn about the incidence and prevalence of carbapenemases in your region.
*A retrospective observational study comparing 14-day mortality between patients with carbapenemase-producing (CP)-CRE compared with non-CP-CRE bacteraemia. There were 83 unique episodes of monomicrobial CRE bacteraemia during the study period: 37 (45%) CP-CRE and 46 (55%) non-CP-CRE.3
†At least 1 antibiotic demonstrating in vitro activity to the isolate recovered within 24 hours of the time the first positive blood culture was obtained; meropenem minimum inhibitory concentrations (MICs) up to 8 µg/mL considered active in vitro as modelling of extended infusion-strategies suggest that reasonable target attainment can be anticipated with MICs up to 8–16 µg/mL.3
‡Active empiric antibiotic therapy was defined as at least 1 antibiotic with in vitro activity, based on antimicrobial susceptibility testing (AST), against the isolate within 24 hours of the time the first positive blood culture was obtained.3
§At least 1 antibiotic demonstrating in vitro activity to the isolate any time after antibiotic susceptibility results were available, up to 7 days after the first positive blood culture was obtained; meropenem minimum inhibitory concentrations up to 8 µg/mL were considered active in vitro (excluding 5 patients in the CP-CRE group and 4 patients in the non-CP-CRE group who died in the first 72 hours, before an antibiotic susceptibility test would have returned).3
ǁActive directed antibiotic therapy was defined as at least 1 antibiotic with in vitro activity against the isolate any time after AST results were available, up to 7 days after the first positive blood culture was obtained.3
¶The primary outcome was 14-day mortality, with Day 1 as the day the first positive blood culture was collected. Fourteen-day mortality was selected as the primary endpoint, as it was thought to be most reflective of death attributable to CRE bacteraemia. Secondary outcomes included 30-day mortality and 30-day recurrence of CRE bacteremia.3
#Adjusting for receipt of active empiric therapy and Pitt bacteraemia score ≥4.3
**A matched case-control study was conducted during a nosocomial outbreak of NDM-1-producers in an adult intensive care unit (ICU) in South Africa. All patients from whom NDM1-producers were identified were considered (n=105). Cases included patients admitted during the study period in whom NDM-1-producing Gram-negative bacteria were isolated from clinical specimens collected ≥48 hours after admission, and where surveillance definitions for healthcare-associated infections were met. Controls were matched for age, sex, date of hospital admission and intensive-care admission. 38 cases and 68 controls were included. Klebsiella pneumoniae was the most common NDM-1-producer (28/38, 74%).10
††Adjusting for comorbid disease.10
‡‡A prospective observational study was conducted in three tertiary-care hospitals located in the Athens metropolitan area; in hospital A, a 500-bed hospital, between February 2004 and March 2006 and in hospitals B and C, 1000- and 400-bed hospitals, respectively, between February 2005 and March 2006. A total of 162 patients were included in the analysis and of the 162 isolates, 67 (41.4%) produced MBL type VIM-1. The endpoint was all-cause mortality at Day 14 after the onset of bacteraemia. Patients discharged from the hospital before Day 14 were considered survivors.6
§§Appropriate empirical antibiotic therapy for an episode of bacteraemia was defined as treatment with at least one antibiotic that had in vitro activity against the infecting organism, initiated within 48 h of the initial positive blood culture, and given in adequate dosage.6
ǁǁA multicentre prospective cohort study at 11 tertiary care facilities in Japan. Patients with isolation of CRE from 1 October 2016 to 31 March 2018 were included in the study. CRE were defined as Enterobacteriaceae with either meropenem MIC ≥2 mg/L or imipenem MIC ≥2 mg/L and cefmetazole MIC ≥64 mg/L. During the study period, 90 isolates (27 carbapenemase-producing Enterobacteriaceae (CPE) and 63 non-CPE) were collected from 88 patients (25 with CPE and 63 with non-CPE) in 9 of 11 hospitals. All CPE were positive for IMP-type carbapenemase, including 11 (40.7%) IMP-11, 6 (22.2%) IMP-42, 4 (14.8%) IMP-6 and 3 (11.1%) each of IMP-10 and IMP-1.11
¶¶Effective empirical therapy was defined as antibiotics given prior to obtaining susceptibility results regarding which CPE/non-CPE were susceptible based on CLSI criteria including carbapenems.11
##Covariates included to generate the propensity score were the following: age-adjusted Charlson combined condition score, leukaemia or lymphoma, dependent functional status, use of nasogastric tube, exposure to a carbapenem within 1 month, polymicrobial isolation and history of admission in the past 3 months. Propensity score adjustments were performed using the stabilized inverse probability weighting. Maximum/median (IQR) standardized bias: (i) unadjusted [0.83/0.21 (0.11–0.37)]; (ii) propensity score-adjusted [0.19/0.08 (0.03–0.11)].11
***Length of stay after isolation of CPE/non-CPE. Excluding cases who died in hospital.11
- Villegas MV, et al. Infectio 2019;23:358–68.
- Budhram DR, et al. Infect Control Hosp Epidemiol 2020;41:37–43.
- Tamma PD, et al. Clin Infect Dis 2017;64:257–64.
- Martin A, et al. Open Forum Infect Dis 2018;5:ofy150.
- Boyd SE, et al. Antimicrob Agents Chemother 2020;64:e00397-20.
- Daikos GL, et al. Antimicrob Agents Chemother 2009;53:1868–73.
- Tan X, et al. Infect Drug Resist 2021;14:125–42.
- Pudpong K, et al. Infect Drug Resist 2022;15:3025–37.
- Daikos GL, et al. Int J Antimicrob Agents 2007;29:471–3.
- de Jager P, et al. PLoS One 2015;10:e0123337.
- Hayakawa K, et al. J Antimicrob Chemother 2020;75:697–708.
- Otter JA, et al. Clin Microbiol Infect 2017;23:188–96.
- Kelly AM, et al. Int J Antimicrob Agents 2017;50:127–34.
- Bakthavatchalam YD et al .In vitro activity of ceftazidime-avibactam and its comparators against carbapenem resistant Enterobacterales collected across India: results from ATLAS surveillance 2018 to 2019. Microbiol Infect Dis. 2022;103:115652.